The axons of sensory neurons invade the epidermal layer of the skin to branch profusely between epithelial cells. Virtually every epithelial cell contacts sensory axons, and every axon contacts many epithelial cells. Our lab has uncovered several interactions between sensory axons and epithelial cells that regulate development and repair. For example, epidermal cells make molecules that guide axons to the skin, clear debris from damaged sensory axons, release factors that promote axon regeneration, and wrap around sensory axons to seal them into ensheathment channels, similar to how glial cells wrap axons. Conversely, we found that sensory axons determine the distribution of cytoskeletal elements, lipids, and adhesion proteins in epithelial cell membranes. Ongoing studies address how axons migrate through the epithelium, how these interactions affect neuronal function, and how innervation impacts epithelial properties.
Projects
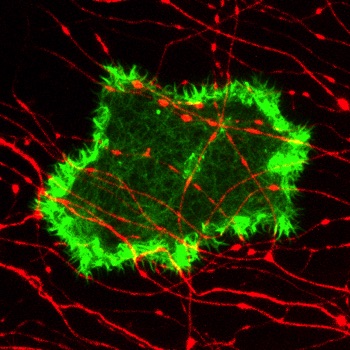
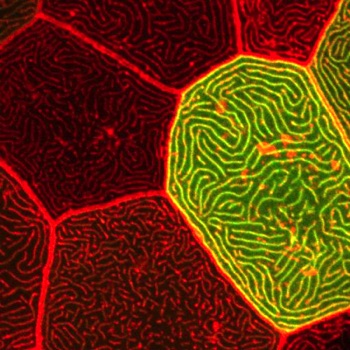
Microridge morphogenesis
Sensory neuron development and repair
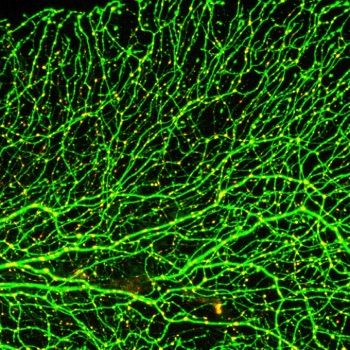
Sensory neurons form elaborately peripheral axon arbors in the skin that detect pain and touch stimuli. Zebrafish sensory axons grow rapidly and are the most superficial and earliest born neurons in the animal, making them ideal for live imaging and addressing universal questions about cellular processes in neurons. Our developmental studies have revealed how axons partition sensory territories in the skin, identified a pathway guiding axons to the skin, revealed how sensory neurons remodel during metamorphosis, and characterized axon caliber dynamics during development. Using a laser to precisely cut axons, we developed methods to study neuronal damage responses in live animals, revealing celllular processes and molecular pathways regulating axon degeneration and regeneration.
Cells make diverse protrusions that endow them with a vast variety of elaborate and beautiful forms. These protrusions are critical for cells to interact with their environment. We study elongated protrusions called microridges, which form stunning maze-like patterns on mucosal epithelial cells, including those in the outer layer of the zebrafish skin. Our studies have revealed how cytoskeletal proteins and mechanical forces regulate microridge formation and organization. We have identified discrete steps in the process of microridge morphogenesis, and several new cytoskeletal regulatory proteins that function at each of those steps. Current projects focus on determining how cortical myosin contraction is regulated in space and time to promote microridge development, and determining how additional cytoskeletal proteins regulate thier morphogenesis.
Neuron-skin cell interactions
Cell migration in the skin
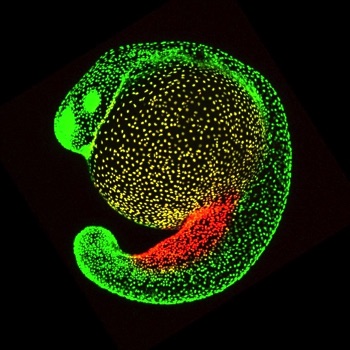
Cell migration is often a mechanism by which cells disperse through tissues. We recently found that two specialized cell types in the skin, mucus cells and ionocytes, disperse by migrating between epithelial cell layers of the epidermis. Mucus cells and ionocytes are cell types crucial for the health of many mammalian mucosal organs, including the lung, kidney, gut, bladder, and ear. Since these cell types adopt a dispersed distribution in all of these tissues, our studies of their development in the zebrafish skin have broad implications. Relatively little is known about how cells migrate through epithelia, so we are using live imaging, molecular manipulations and transcriptomic studies to learn more about the motility of these cells. We are also studying how sensory axons and surrounding epithelial cells impact the migration of mucus cell and ionocyte precursors.