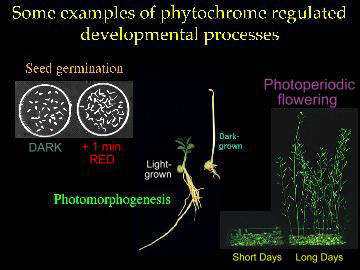
Photoreceptors and circadian clocks are universal mechanisms for sensing and responding to the light environment. In addition to regulating daily activities, photoreceptors and circadian clocks are also involved in the seasonal regulation of processes such as flowering. Circadian rhythms govern many plant processes, including movements of organs such as leaves and petals, stomata opening, stem elongation, sensitivity to light of floral induction, metabolic processes such as respiration and photosynthesis and expression of a large number of different genes. There is an intimate relationship between certain photoreceptors and the circadian clock. In plants, phototransduction not only sets the phase, but also affects the amplitude and period of circadian rhythms. Members of the phytochrome family of plant photoreceptors, which can exist in two photochemically interconversible forms (Pr and Pfr) and are involved in regulation of plant development and growth, play important roles in regulating clock activities.

Our long term goal is to understand in detail how the phytochrome system interacts with the circadian clock to regulate the many developmental processes of higher plants. Our strategy has been to focus on latter steps of the light signal transduction chain, wherein phytochrome acts in concert with the circadian clock to alter the expression of specific genes, using examples of light-regulated genes in Arabidopsis thaliana.
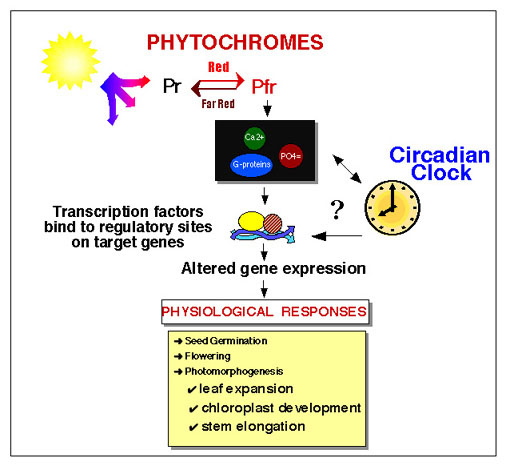
The Lhcb gene family (also designated as CAB genes), which encodes apoproteins of the light-harvesting complex associated with photosystem II, has been one of the model systems for studies of both phytochrome and circadian regulation of gene expression. These studies have led to the identification of promoter elements involved in phytochrome induction and circadian rhythm of gene expression. Several proteins interacting with some of these regions have been identified but until recently, in vivo function had not been demonstrated for any of them.
We have isolated and characterized a transcription factor, called CCA1, which binds to a region of an ArabidopsisLhcb promoter that is necessary for its phytochrome responsiveness ( Wang et al., 1997 ). CCA1 is a Myb-related protein that binds to at least two of the Lhcb genes of Arabidopsis, at a sequence that is conserved in Lhcb genes of many species. Lines of transgenic Arabidopsis plants expressing antisense RNA for CCA1 showed reduced phytochrome induction of the endogenous Lhcb1*3 gene. Therefore, CCA1 appears to be a key element in the functioning of the phytochrome signal transduction pathway leading to increased transcription of this Lhcb gene in Arabidopsis.
The Circadian Clock Associated 1 (CCA1) gene is closely associated with the circadian oscillator of higher plants[Wang ZY, Tobin EM. Cell 1998 Jun 26;93(7):1207-1217]. |
The protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein[Sugano S, Andronis C, Green RM, Wang ZY, Tobin EM. Proc Natl Acad Sci U S A 1998 Sep 1;95(18):11020-11025]. |
CCA1 is a Myb-related transcription factor that is important for the phytochrome regulation of light-harvesting chlorophyll protein genes (Lhcb or CAB genes) [Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM. Plant Cell 1997 Apr;9(4):491-507]. Further investigation of the function of CCA1 has resulted in the Tobin lab finding that it plays a central role in circadian rhythms in the plant.
CCA1 is itself expressed with a circadian rhythm.
Overexpression of CCA1 in transgenic Arabdiopsis plants abolished the circadian rhythms of several genes with dramatically different phases.
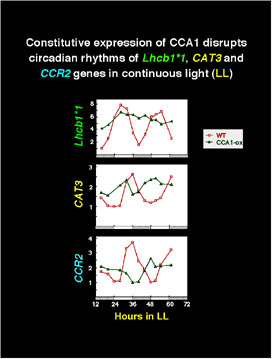
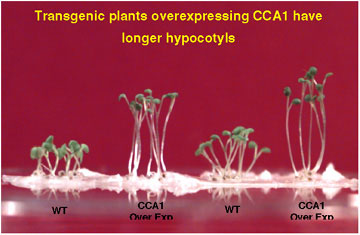
These plants also had longer hypocotyls and delayed flowering, developmental processes regulated by light and the circadian clock. Furthermore, leaf movement rhythms were abolished.

The expression of both endogenous CCA1 and the related LHY gene was suppressed in the CCA1 overexpressing lines, suggesting a feedback supression regulation of transcription.
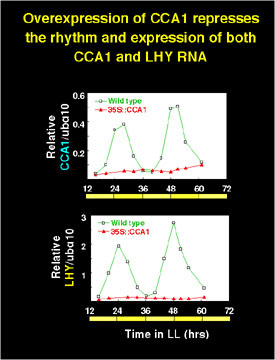
The evidence suggests that CCA1 is a part of a feedback loop that is closely associated with the circadian clock in Arabidopsis, and may represent the central oscillator itself.

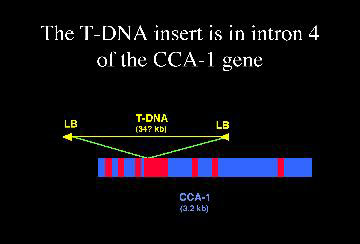
By screening pools of DNA from lines of plants with random T-DNA insertions, we identified a line in which the CCA1 gene was interrupted.
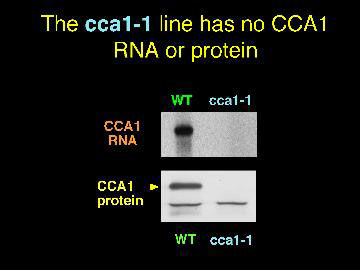
With no CCA1 protein, phytochrome induction of Lhcb RNA is reduced to 60% of the normal level. Perhaps the closely related LHY gene is responsible for the remaining induction.
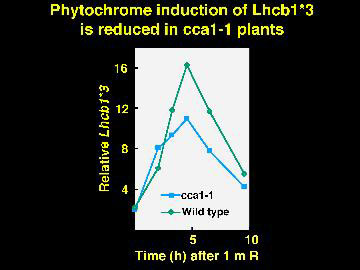
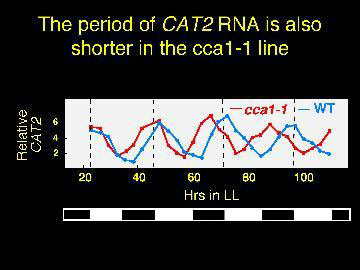
Plants with no CCA1 have the periods of gene expression shorted by about three hours.
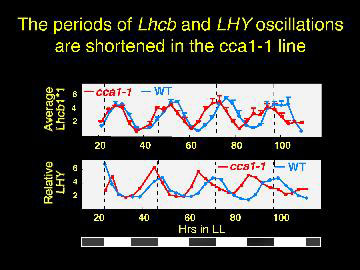
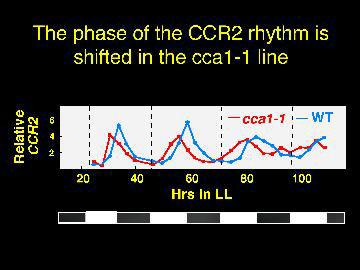
The CCR gene may be phase shifted, suggesting the possibility of a second oscillator.
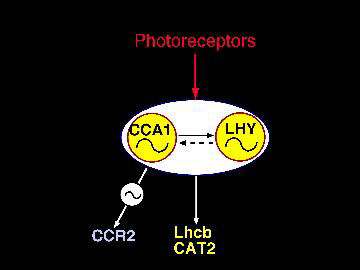
Possible roles and interaction of CCA1 and LHY.

[Sugano S, Andronis C, Green RM, Wang ZY, Tobin EM. The protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc. Natl. Acad. Sci. U S A 1998 Sep 1;95(18):11020-11025].
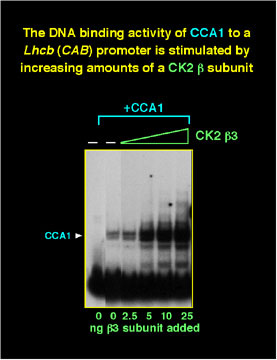
A yeast interaction screen was used to identify a protein that interacts with CCA1. This protein encodes a novel regulatory (beta) subunit of the protein kinase CK2 which was designated CKB3. It is the only reported example of a third beta-subunit of CK2 found in any organism. CK2 beta-subunits stimulate binding of CCA1 to its binding site on a Lhcb (Cab) gene promoter, and recombinant CK2 is able to phosphorylate CCA1 in vitro.
The formation of a DNA-protein complex containing CCA1 from plant extracts depends on phosphorylation, and, if CK2 activity in the extracts is inhibited, the complex cannot be detected in vitro.
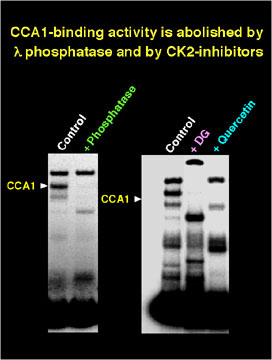
The results suggest that CK2 can modulate CCA1 activity both by direct interaction and by phosphorylation of the CCA1 protein and that CK2 may play a role in the function of CCA1 in vivo.